In Situ Chemical Oxidation
On this page:
- Schematic
- Introduction
- Other Technology Names
- Description
- Development Status
- Applicability
- Cost
- Duration
- Implementability Considerations
- Resources
Schematic
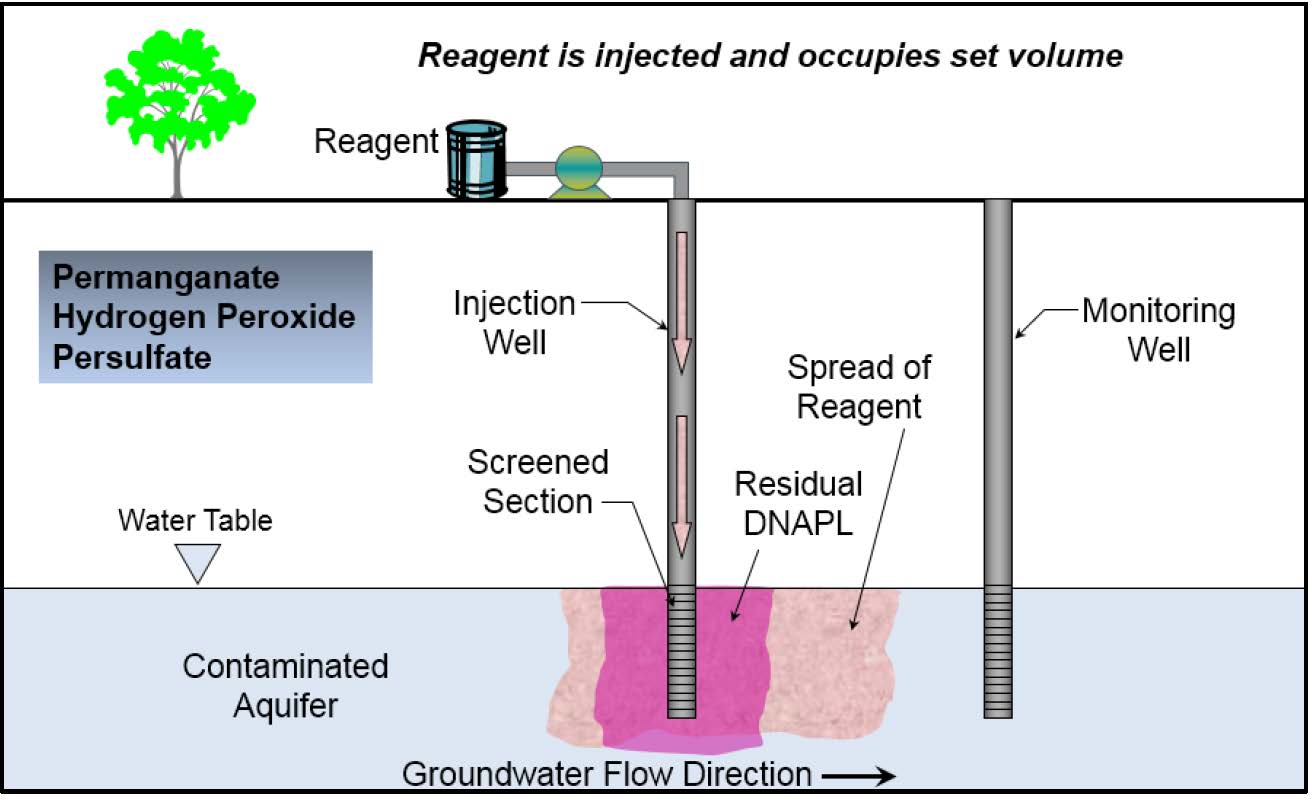
This information may be reproduced without restriction as long as the source attribution is included.
In Situ Chemical Oxidation
Introduction
In site chemical oxidation (ISCO) is an in situ remediation technology that involves the injection of chemical oxidants into the subsurface, the most common of which are permanganate, persulfate, and hydrogen peroxide. ISCO is applicable to treat a wide-range of contaminants of concern (COCs) including chlorinated ethenes and ethanes, petroleum hydrocarbons and their constituents, 1,4-dioxane, energetics, pesticides, and phenols among others. ISCO also can facilitate the removal of non-aqueous phase liquids (NAPLs). It is important to select an appropriate oxidant, dosing, and activation agent (if needed) based on the COCs present since not all compounds can be treated effectively by a single oxidant.
Other Technology Names
ChemOx
Chemical oxidation
Description
ISCO is a remediation technology involving the injection and distribution of a chemical oxidant into the subsurface to transform COCs in groundwater and soil into innocuous byproducts. It can be considered for contaminant mass removal at sites where groundwater and/or porous media contain COCs such as petroleum hydrocarbons, chlorinated solvents, 1,4-dioxane, and energetic compounds, which are amenable to oxidation.
Oxidants
A variety of ISCO reagents are available on the market including hydrogen peroxide, sodium persulfate, potassium permanganate, sodium percarbonate, potassium permanganate, and sodium persulfate, which generally are applied in liquid form. 1 Oxidants normally shipped in solid form, such as potassium permanganate or sodium persulfate, are dissolved easily and mixed on site to form a solution having the required design concentration. Design considerations for the common oxidants are listed in the table below. The three most common oxidants are described in the paragraphs that follow.
Permanganate
Permanganate has been used for more than 50 years to treat contaminants in drinking water and wastewater. More recently, permanganate has been used as an oxidant to treat contaminated soil and groundwater. When permanganate reacts with organic contaminants, the contaminants are oxidized to carbon dioxide and the permanganate is reduced to a manganese dioxide salt and a potassium (or sodium) salt.
The reaction of permanganate is described by second order kinetics. The rate of reaction is dependent upon the concentrations of both the permanganate and the COCs as well as the concentrations of other competing species, such as reduced metals and natural organic matter. Hence, increasing the concentration of permanganate will increase the rate of reaction with the COCs and other competing species. Also, as the concentration of COCs are reduced over time, the rate of reaction decreases. Because permanganate reacts slowly in the aquifer, it is able to travel further from the injection point before it completely reacts compared to other oxidants.
Either potassium permanganate (KMnO4) or sodium permanganate (NaMnO4) can be used. KMnO4 is a solid and its solubility in water is limited to 6% [wt/wt (SERDP/ESTCP, 2011)]. It is easily transported to the field where it can be mixed with potable water or groundwater to the desired concentration. KMnO4 dust can be a health hazard that requires some control during mixing on site. Although KMnO4 costs less than NaMnO4, it may require sophisticated equipment on site for dissolving and distributing. NaMnO4 has a higher solubility in aqueous solution at approximately 40% (SERDP/ESTCP, 2011). It is typically transported to the site as a liquid and then diluted to the desired concentration. NaMnO4 is more reactive in the presence of certain reductants and needs to be handled carefully.
Field applications of permanganate have generally consisted of 0.5 to 3.0% concentration of injected solution. Higher concentrations can be used, but sometimes lead to excessive manganese dioxide formation around injection points, which can clog both the injection point and the aquifer.
Persulfate
Persulfate (S2O82-) is delivered in the form of sodium persulfate. Sodium persulfate has a water solubility of approximately 40% and results in a clear solution. It is very reactive and produces innocuous byproducts.
Persulfate reaction chemistry is complex. Oxidation can occur via electron transfer or free-radical pathways. It is most common to activate persulfate using elevated temperatures (35 to 40°C), creating strongly alkaline conditions (pH upwards of 10 or 12), with ferrous iron (Fe[II]), or with hydrogen peroxide (H2O2) to generate sulfate free radicals, which facilitate contaminant destruction.
Sodium persulfate is highly soluble (73 g/100 g H2O at 25 °C), and the density of a 20 g/L solution at 25°C (1.0104 g/mL) is greater than water. Once injected, a highly-concentrated solution of Na2S2O8 can be transported by density-driven and diffusive transport. In addition, it is stable in the subsurface, with half-lives of up to 700 days reported in the literature (SERDP/ESTCP, 2011). It has less affinity for natural organic matter than permanganate or peroxide; hence, less oxidant is required. Persulfate can oxidize benzene, while permanganate cannot. This allows persulfate to be used in the remediation of fuel spills and benzene, toluene, ethylbenzene, and xylene (BTEX)-contaminated groundwater. The generation of sulfate also can promote biodegradation of petroleum constituents under anaerobic conditions, which can be designed as a polishing step after ISCO is completed. Persulfate does not produce the heat and gas evolution involved with hydrogen peroxide application.
Hydrogen Peroxide
It has been recognized since the late 1800s that hydrogen peroxide in the presence of an iron catalyst creates a very strong oxidant, commonly known as Fenton's reagent or catalyzed hydrogen peroxide (CHP). Although hydrogen peroxide itself is a strong oxidizer that is capable of oxidizing organic compounds, when combined with ferrous iron, the iron serves as a catalyst to form highly oxidizing hydroxyl radicals, which have an oxidation potential twice that of hydrogen peroxide. The process consists of a number of reactions, which are complex and consist of a variety of initiating, propagating, and terminating steps. These reactions produce several types of free radicals including the hydroxyl radical (OH•), the perhydroxyl radical (HO2•), and the superoxide anion (O2•-), all of which have very high oxidation potentials. The free radicals oxidizes organic compounds into smaller hydrocarbons that are easily oxidized to carbon dioxide and water.
The optimal pH for the generation of hydroxyl radicals and for hydrogen peroxide stability is between 3.5 and 5.0. An acid, such as sulfuric or hydrochloric, is often added to the aquifer to create a desired acidic pH. Alternatively, a chelating agent, such as sodium citrate, citric acid, or ethylenediamine tetra acetic acid (EDTA) can be added to control the rate of decomposition of hydrogen peroxide allowing the reaction to occur at a near neutral pH. CHP systems are designed to either introduce an iron catalyst, such as ferrous sulfate or ferric chloride, or to take advantage of the naturally occurring minerals in the subsurface. Common minerals such as goethite or hematite among others, have been documented to effectively catalyze the peroxide reaction (SERDP/ESTCP, 2011). Low loadings may not provide sufficient iron to initiate catalysis of the hydrogen peroxide, whereas high concentrations can reduce aquifer permeability and the rate of reactions that terminate the formation of the hydroxyl free radical. The required dose is based on a number of site- and application-specific factors including, but not limited to, the presence of naturally occurring iron in soil and groundwater, concentrations of organic matter and COCs, and the target concentration of hydrogen peroxide in the aquifer. Optimum concentrations are best determined by performing bench-scale treatability studies.
CHP can be used to oxidize both contaminated soil and groundwater. CHP systems have been designed to introduce concentrations of peroxide ranging from 0.5 to 12%. Concentrations at the lower end of the range (i.e., 0.5 to 1%) are used to treat dissolved-phase contamination, whereas higher concentrations are required at sites where sorbed or non-aqueous phase liquid (NAPL) is present. It is important to note that concentrations greater than about 10% can be problematic due to the exothermic nature of the reaction, which can significantly raise the temperature of the soil and create fire and explosion hazards. In addition, a substantial volume of gas can be generated that may cause surfacing of groundwater and reagents and potentially mobilize contaminants. Additional precautions and rigorous monitoring should be employed when using high concentrations.
Oxidant Delivery Approaches and Design Considerations
Delivery methods for liquid oxidantsinclude direct push methods through wells or points and groundwater recirculation through horizontal or vertical extraction and injection wells. Combined approaches consisting of direct push in some locations and recirculation in others are sometimes used. Hydraulic fracturing can be used at sites where introduction into low permeability media is required. Regardless of the method used, repeat additions of the oxidant and any activation catalysts (e.g., ferrous sulfate, NaOH, etc.) typically are necessary to obtain substantial oxidation of the organic contaminant and achieve remedial goals.
Direct push methods are well-suited for consolidated materials and weathered bedrock because there tends to be sufficient interconnected pore space to permit the distribution of the amendment throughout the treatment zone. In low permeability materials, such as silt and clay, the radius of influence (ROI) may be limited and high pressure may develop and can compromise the integrity of the formation. In addition, there is a greater likelihood to displace contaminated groundwater compared to recirculation approaches. Application of oxidants using a direct injection approach typically occurs over a short time (minutes to hours) at each location. However, in some cases, it may be desirable to design systems using a gravity feed system to utilize gravity to continuously introduce the amendments into wells over an extended time. Temporary or permanent vertical wells can also be used for direct injection of liquid oxidants under low pressure or gravity feed. Wells are used for deeper applications (beyond 30 -50 feet), to reduce long-term delivery costs when multiple injection events are projected, or for treatment of bedrock geology.
Groundwater recirculation (injecting from certain wells and extracting from others) can be used to overcome some of the distribution challenges associated with direct injection. Recirculation systems are designed to extract groundwater, add and mix the reagents and substrates, and reinject the amended water into the aquifer. Regulatory requirements may require above-ground treatment of dissolved contaminants prior to re-injection. Recirculation and mixing of amendments into groundwater is commonly performed using permanent injection and extraction wells, although a combination of direct push points and permanent wells can be used. Recirculation of amended groundwater can be used to more cost effectively treat larger and deeper contaminated areas, especially involving more transmissive aquifer units. Systems also can be designed to facilitate distribution in heterogeneous and lower permeability aquifers where long-term matrix diffusion rebound is a concern. Recirculation minimizes displacement of contaminated groundwater compared to direct injection systems by creating flow pathways from the injection locations to the extraction locations. Some applications perform recirculation until a pre-determined pore volume of groundwater has been exchanged in the aquifer. The goal of this approach is to increase long-term distribution and persistence of the amendments throughout the treatment area and enhance dissolution of the DNAPL if present.
Solid amendments, such as permanganate, can be used to treat vadose zone soils. Solid oxidants can be mixed into vadose zone soils using various types of specially equipped backhoes or large diameter auger equipment. Introduction of solid amendments into low permeability media can be enhanced using pneumatic fracturing techniques.
Understanding the characteristics of the subsurface is important when applying oxidants in situ. Distribution tends to be easier in sandy, more permeable and homogeneous aquifers. It is difficult to obtain adequate delivery volume and distribution of the oxidant in fine sand, silt and clayey materials and in heterogeneous aquifers containing multiple discrete lithologic units. In less permeable aquifers, injection points must be placed closer together. Geochemical conditions including hardness, total organic carbon, dissolved organic carbon, pH, and contaminant concentration must be known in order to design the amendment distribution system and determine appropriate mass, concentration, and flowrate of amendments.
The concentration of oxidant used depends on the concentrations of contaminants and organic matter as well as the type of delivery approach applied. Typically, lower concentrations of oxidant can be used when injected via a recirculation approach, since oxidant is being continuously delivered to the treatment area, and higher concentrations can cause rapid deterioration of the delivery equipment. Higher oxidant concentrationsneed to be used for direct injections, since it is being introduced on a batch basis, and it may be desirable to minimize the volume of fluid injected to reduce the chance of displacing contaminated groundwater.
Monitoring and Data Interpretation
A variety of process monitoring must be performed while introducing and distributing oxidants and any associated activation agents. The flowrate, pressure, and temperature are measured at the injection manifold and/or each injection well/point to control injection delivery. Similar data are collected from the extraction well if a recirculation system is employed.
Several indicator parameters should be measured at a representative number of performance monitoring locations when injecting permanganate, persulfate, and hydrogen peroxide into the subsurface. These include groundwater quality parameters such as pH, conductivity, oxidation-reduction potential; groundwater elevations; and concentrations of potentially mobilized metals in groundwater (conversion of trivalent to hexavalent chromium is of particular concern for oxidation). These parameters are measured to evaluate distribution of the oxidant, as well as to determine hydrogeologic and geochemical changes, which may occur in the aquifer as a result of the oxidant application. Although these changes typically occur within the treatment area and return to near baseline values shortly after the application, the aquifer geochemistry should be monitored to ensure that any nearby downgradient receptors are not impacted. A semi-qualitative evaluation of the concentrations of oxidantscan easily performed in real time using field tests kits and/or a field spectrophotometer. The presence of permanganate in wells also can be determined by visual inspection (water becomes purple). Similarly, evidence of bubbles in groundwater and/or elevated groundwater temperatures may be indicative of the presence of peroxide in the vicinity of a monitoring well.
After each oxidant application is complete, longer-term performance monitoring should be performed (NAVFAC, 2015). This monitoring consists of measuring concentrations of COCs in monitoring wells at various time intervals to determine the degree of treatment and the need for additional injections. Periodic monitoring should be performed involving the same indicator parameters discussed above to assess whether the aquifer has returned to baseline conditions and any mobilized metals attenuate before reaching any sensitive receptors. Proper preservation procedures for groundwater need to be followed to neutralize any resdiual oxidant presence in collected groundwater samples to avoid ongoing reactions that can bias COC results low.
Development Status and Availability
The following checklist provides a summary of the development and implementation status of ISCO:
☐At the laboratory/bench scale and shows promise
☐In pilot studies
☒At full scale
☒To remediate an entire site (source and plume)
☐To remediate a source only
☒As part of a technology train
☐As the final remedy at multiple sites
☐To successfully attain cleanup goals in multiple sites
ISCO is available through the following vendors:
☒Commercially available nationwide
☐Commercially available through limited vendors because of licensing or specialized equipment
☐Research organizations and academia
Applicability
|
Contaminant Class Applicability Rating for ISCO (Rating codes: Demonstrated Effectiveness, ◐ Limited Effectiveness, No Demonstrated Effectiveness, I/D Insufficient Data, N/A Not Applicable) | ||||||||
|---|---|---|---|---|---|---|---|---|
Nonhalogenated VOC |
Halogenated VOC |
Nonhalogenated SVOC |
Halogenated SVOC |
Fuels |
Inorganics |
Radionuclides |
Munitions |
Emerging Contaminants |
| ● | ● | ● | ● | ● | ○ | ○ | ● | I/D |
Regarding emerging contaminants, ISCO can be effective in-situ treatment method for 1,4-dioxane. Research into the treatment of PFAS by oxidation is still at a research stage. Initial bench-scale testing of oxidation of PFAS have yielded mixed results (e.g., shown to degrade PFOA, but not PFOS), with heat activated persulfate showing the most promise to date.
A list of specific compounds and the degree to which they are treated (E = excellent, G = good, P = poor) is provided in the table below. Permanganate, persulfate, and peroxide are well suited for application across a wide range of conditions; however, COCs and other site-specific conditions (e.g., soil lithology and hydrology, aboveground and belowground structures, nearby receptors) and RGs must be carefully considered when selecting the most appropriate oxidant for a site.
| Oxidant Applicability to Specific Contaminants (E = excellent, G = good, P = poor) |
|||
| Contaminant | Oxidant | ||
| Permanganate | Activated Persulfate | Activated Peroxide | |
| Petroleum hydrocarbons | G | E | E |
| Benzene, toluene, ethyl benzene, xylenes | E | E | E |
| Phenols | G | E | E |
| Polycyclic aromatic hydrocarbons | G2 | E | E |
| Methyl tertiary butyl ether | P | E | E |
| Tert-butyl alcohol | NA | E | E |
| Chlorinated ethenes | E | E | E |
| Carbon tetrachloride | P | G | P |
| Chloroform | P | G | P |
| Methylene chloride | P | G | G |
| Chlorinated ethanes | P | G | G |
| Chlorobenzene | P | E | E |
| Polychlorinated biphenyls | P | P | P |
| Energetics (RDX, HMX, TNT) | E | G | G |
| Pesticides | G | G | P |
| 1,4-dioxane | P | E | E |
NA - Not available
Source: Adapted from: In Situ Chemical Oxidation, EPA Engineering Issue Paper, Scott G. Huling and Bruce E. Pivets. August 2006.
Cost
ISCO can be a very cost-effective technology if properly designed and applied. Similar to many in situ remediation technologies, the most critical cost factors are associated with the contaminant mass to be treated, the nature and extent of contamination (i.e., size of the treatment area), and number of injection points/wells required. As with all in situ technologies, application costs vary according to site conditions and contaminants. Major cost drivers include:
Upfront Costs
- Need for bench-scale tests and/or pilot studies to demonstrate effectiveness at a particular site and determine relevant design parameters.
- Quantities of oxidant and activators required, which are dictated by the contaminant type and mass to be treated, type of oxidant used, treatment area, and site geochemistry.
- Lithology and hydrogeology: In particular, native SOD may vary from site to site, and this can affect oxidant consumption and ROI around injection points.
- Number of injection points/wells required, which is dictated by the contaminant mass to be treated, site hydrogeologic conditions, and area to be treated.
- Equipment (including need for permeability enhancement techniques) and labor to introduce and distribute the amendments, which is dictated by the application method and duration of each injection event.
- Areal extent of contamination and depth of contamination.
- Presence of above and below ground structures and utilities.
Operation and Maintenance Costs
- Frequency of reapplication of amendments. Normally two or more injection events are required (SERDP/ESTCP, 2011). However, site specific remedial cleanup goals (RGs) and levels contamination (e.g., presence of NAPL) can significantly impact the number of applications required. Sites with low permeability soil units are subject to matrix diffusion rebound, which can drastically influence the number of injection events or ability to meet RGs given the shorter persistence times for ISCO amendments.
- Monitoring requirements after amendment addition.
- Treatment timeframe.
The list above highlights those cost dependencies specific to composting and does not consider the dependencies that are general to most remediation technologies. Click here for a general discussion on costing which includes definitions and repetitive costs for remediation technologies. A project-specific cost estimate can be obtained using an integrated cost-estimating application such as RACER® or consulting with a subject matter expert.
Duration
The duration of the field construction or amendment application for ISCO is relatively quick, ranging from a few days to a few weeks. This short duration is one of the attractive features of ISCO, especially at sites where disruption of ongoing facility operations is a concern.
The persistence of the oxidants in the aquifer after completing active injection varies based on the oxidant type, the concentration injected, and site-specific lithology and hydrogeology. Depending on SOD, permanganate can be the longest lasting oxidant, possibly persisting for up to a year at a site depending on concentrations injected and groundwater flow. Hydrogen peroxide is the least persistent of the oxidants described, generally lasting from a few days to one to two weeks. Persulfate typically persists for weeks to months, and is less influenced by SOD than permanganate or peroxide. Primary factors that influence the duration of the ISCO technology include:
- RGs
- Treatment methodology (i.e., source area treatment, dissolved plume treatment, containment)
- Presence of NAPL
- Number of injection events required
- Concentration of amendments applied
Most ISCO treatment applications require multiple injection events (typically three on average) to achieve site RGs (or treatment to the extent practicable). Treatment timeframes, however, can be decades for more complex remediation sites involving significant source area contaminant mass, large plumes, and/or high initial dissolved phase contaminant concentrations. Because of the relatively short amendment persistence and resulting susceptibility to matrix diffusion rebound, ISCO can require more frequent injection events than other in situ amendment technologies. Once a point of diminishing returns is reached, the remedy may need to be transitioned to an alternative less-aggressive technology such as enhanced in situ bioremediation or monitored natural attenuation (MNA) and the groundwater monitored for contaminant rebound. Since it is often difficult to attain drinking water RGs in groundwater through ISCO treatment alone, the duration will also be dependent on the interim objectives established for the termination of injections and transition to an alternative remedial approach.
Implementability Considerations
The following are key considerations associated with applying ISCO:
- A primary limitation of ISCO relates to achieving adequate distribution and contact of the reagent with the COCs. Care must be taken to carefully design the injection system so that adequate contact is achieved. The ROI for ISCO reagents may be limited due to the reactive nature of the oxidants. Modeling using a reactive transport model can help address aquifer changes as the oxidant reacts with the COCs and aquifer materials. In general, a typical ROI for ISCO reagents may range from about 5 to 15 feet per delivery point.
- If an activating agent is used (i.e., iron, heat, alkaline, etc.), monitoring should be performed to gauge the distribution and ROI of the activating agent to ensure that the reagent is activated according to design. Activators may not be distributed the same distance as the oxidants due to different reaction rates, retardation factors, etc. In some cases, sequential application of the activator and oxidant may be more desirable than to apply the oxidant and the activator at the same time to minimize unproductive consumption of the reagents and alleviate health and safety issues.
- Site lithology and hydrogeology impact the technology's ability to achieve effective contact between the reagents and COCs. Heterogeneous soil lithology can limit the distribution of ISCO amendments, which can result in pockets of untreated residual contaminant mass throughout the treatment area. An aquifer hydraulic conductivity of 10-5 cm/sec or greater facilitates effective distribution.
- Soils should preferably have a low clay content to allow oxidant penetration.
- Moderate to high levels of immobilized NAPL and residual contaminant mass may be more cost effectively treated by other in situ technologies (e.g., in situ thermal treatment).
- Preferential flow paths can severely influence amendment distribution and result in pockets of untreated contaminant mass. Amendment distribution becomes more difficult and may be infeasible for lower permeability clay, highly layered, or heterogeneous subsurface environments. Higher injection pressures or permeability enhancement techniques (e.g., hydraulic or pneumatic fracturing) can improve distribution of amendments for these conditions.
- Change in oxidation states and/or pH can mobilize metals within the treatment zone.
- Permits are usually required to receive permission from state and federal agencies to conduct ISCO. The permits that may be required include a waiver of the underground injection control (UIC) requirements and an air quality permit if emissions are expected from exothermic reactions and heat generation.
In addition to the above-mentioned issues, there are a number of oxidant-specific considerations that are summarized in the table below.
| Implementability Considerations for the Application of ISCO Amendments (adapted from NAVFAC, 2013 & 2015) |
|
| ISCO Reagent | Injection/Distribution Design Considerations and Challenges |
| Permanganate |
|
| Persulfate |
|
| Hydrogen Peroxide |
|
Resources
EPA. A Citizen's Guide to Chemical Oxidation (2001)
This fact sheet provides a brief overview of chemical oxidation and its benefits.
EPA. Engineering Issue: In-situ Chemical Oxidation (2006)
The engineering issue summarizes the fundamentals of ISCO remediation technology based on peer reviewed literature, EPA reports, etc.
ESTCP. In Situ Chemical Oxidation for Remediation of Contaminated Groundwater: Summary Proceedings of an ISCO Technology Practices Workshop Project ER 0623 (2008)
This project provides several documents that help site managers – site specific engineering and application, case histories and database, supplemental information and tools and frequently asked questions guide.
ESTCP. In Situ Chemical Oxidation for Groundwater Remediation (2011)
Environmental Remediation Technology
This book presents detailed design guidance and scientific basis for the application of ISCO.
ITRC. Technical and Regulatory Guidance for In Situ Chemical Oxidation of Contaminated Soil and Groundwater. Second Edition (2005)
The guidance document outlines chemical oxidation technologies and their delivery techniques – hydrogen peroxide, potassium and sodium permanganate, sodium persulfate and ozone.
NAVFAC. In Situ Chemical Oxidation Fact Sheet
This fact sheet provides an overview of the ISCO technology and lessons learned during its implementation.
NAVFAC. In Situ Chemical Oxidation (ISCO) Web Tool
This tool presents the current state of ISCO technology, a discussion of how each type of ISCO chemical works, and case studies that showcase their application. This technology involves the subsurface delivery of a chemical oxidant to destroy organic contaminants, reducing potential risks to public health and the environment.
NAVFAC. Cost and Performance Report for Persulfate Treatability Studies (2010)
The report presents five persulfate application projects at four Navy sites and one Marine Corps site. It provides lessons learned and other performance-related information under different site conditions.
NAVFAC. RITS on In Situ Chemical Oxidation (2010)
This presentation provides an introduction to ISCO, its design, implementation and monitoring practices. In addition, it includes a discussion on case studies.
NAVFAC. Best Practices for the Injection and Distribution of Amendments (2013)
This guidance document provides a variety of best practices to effectively distribute amendments for technologies including ISCO, in situ enhanced bioremediation, and in situ chemical reduction.
NAVFAC. Design Consideration for In Situ Chemical Oxidation (2015)
Provides a summary of best practices for ISCO design, tips for appropriate quality assurance and quality control (QA/QC) measures.
Strategic Environmental Research and Development Program (SERDP)/Environmental Security Technology Certification Program (ESTCP). In Situ Chemical Oxidation for Groundwater Remediation (2011)
R.L. Siegrist, M. Crimi, and T.J. Simpkin (eds.). Springer, New York. SERDP ESTCP Environmental Remediation Technology, Vol. 3, ISBN 978-1-4419-7825-7, 678 pp, 2011
Provides principals and best practice for design, application, and monitoring of in situ chemical oxidation remedies.
SERDP. Using Electrical Resistivity Imaging to Evaluate Permanganate Performance During In Situ Treatment of an RDX-Contaminated Aquifer ER-200635 (2009)
The project demonstrates the use of electrical resistivity imaging (ERI) to monitor and facilitate an ISCO demonstration designed to treat RDX with permanganate at the Nebraska Ordnance Plant.
SERDP. Improved Understanding of In Situ Chemical Oxidation (ISCO) ER-1289 (2010)
This project provides improved understanding of ISCO applicability and optimized deployment conditions through two separate bench-scale efforts.
Ozone also is used at some sites. However, ozone is introduced as a gas, which requires a different design and method of introduction and associated challenges. Hence, it is not covered in this technology profile. ↩
Ozone also is used at some sites. However, ozone is introduced as a gas, which requires a different design and method of introduction and associated challenges. Hence, it is not covered in this technology profile. ↩
Effective for naphthalene, phenanthrene, and pyrene ↩
Effective for naphthalene, phenanthrene, and pyrene ↩
